Introduction
In newly diagnosed multiple myeloma (NDMM) patients eligible for autologous stem cell transplantation (ASCT), daratumumab plus, bortezomib, thalidomide, and dexamethasone (D-VTd) followed by high-dose melphalan with ASCT, is considered standard of care in Europe. The CASSIOPEIA trial reported a lower stem cell (SC) yield and higher plerixafor (PLX) use, but no impact over ASCT feasibility and safety for the D-VTd group. However, real-world data from clinical experience after its approval is scarce.
In the United Kingdom, the National Institute for Health and Care Excellence approved D-VTd as frontline treatment in November 2021. Our aim was to describe the real-world experience regarding mobilization, harvest, and ASCT outcomes in NDMM patients receiving D-VTd.
Methods
We included all consecutive NDMM patients referred from 10 haematology centres to our institution and subsequently to apheresis, from June 2022 to May 2023. Patients received D-VTd as induction and had achieved at least partial response at time of referral. Patients underwent SC mobilisation with cyclophosphamide 1.5g/m 2 D+1, GCS-F 5µg/kg D+5 to D+10, and apheresis D+11 and D+12. Additional VTd cycles were given when length of waiting for apheresis was more than 6 weeks. Pre-emptive PLX was given for pre-apheresis CD34+count between 5-15cells/µL. After a successful harvest (≥2.0x10 6 CD34+cells/kg) ASCT was scheduled in 4-6weeks.
Results
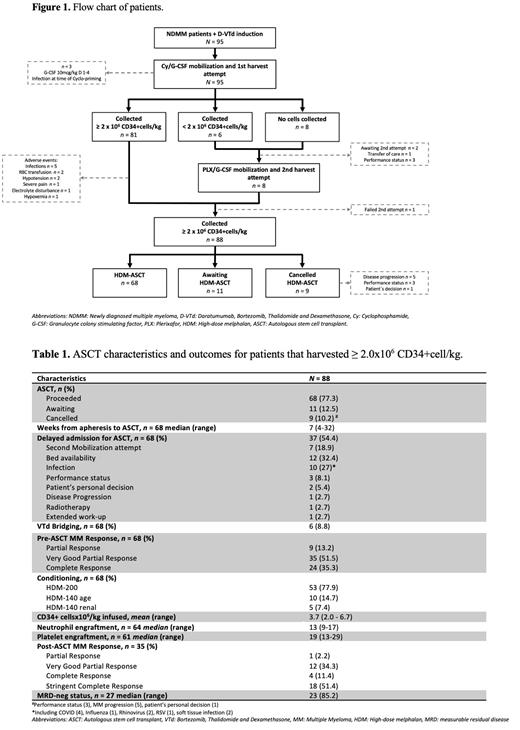
A total of 95 patients were included. The median age was 63 years (range 34-74), 71.6% were male, 16.8% were ISS-III, 9.2% were R-ISS-III, and 28.9% had high-risk cytogenetics. Regarding induction treatment patients received 4 cycles of D-VTD; additionally 11 (11.6%) received radiotherapy, and 36 (37.9%) additional VTD pre-apheresis,th a median of 2 (range 1-4). MM response pre-apheresis was ≥VGPR in 80%.
The median time from the start of D-VTd to apheresis was 6 months (range 4-10). A total of 87 patients underwent apheresis; 21 (22.1%) received pre-emptive PLX and 71 (81.6%) underwent two apheresis procedures. The mean pre-apheresis and total dose CD34+cells were 29.2/µL (range 0.4-220.7) and 4.5x10 6/kg (range 1.2-12.6), respectively. From the 14 patients that failed to collect ≥2.0x10 6cell/kg on first attempt, 8 underwent a second attempt. Overall harvest success rate was 92.6% ( n = 88) (Figure 1). Regarding the additional VTd versus non-VTd groups, mean total CD34+cell dose was 5.1x10 6/kg (range 1.3-10.7) versus 4.8x10 6/kg (range 1.2-12.6) ( p = 0.61) and PLX was used in 25% versus20.3% ( p = 0.28).
After a median of 7 weeks (range 4-32), 68 patients (77.3%) proceeded to ASCT, 11 (12.5%) are awaiting, and 9 (10.2%) were cancelled. A 54.4% (n = 37) of patients experienced a delay for ASCT, 7 due to second harvest attempt. Mean CD34+cells infused was 3.7x10 6/kg (range 2.0-6.7). All patients achieved neutrophil and platelet engraftments after a median of 13 days (range 9-17) and 19 days (range 13-29), respectively. One patient died after engraftment, day +49, due to pneumonia. MM response at 100-days after ASCT was ≥VGPR in 97.1% and MRD-neg in 85.2% of the evaluated patients (Table 1).
Conclusions
Based on our results, we confirm that compared to published data the addition of daratumumab to VTd in NDMM patients, results in a lower SC yield and higher pre-emptive PLX administration. As expected, when reflecting the real-world practice timely interventions are difficult to maintain leading to delays on harvesting and ACST procedures. Herein we report the implementation of a “bridging” strategy with additional VTd cycles between D-VTd induction and apheresis, this did not influence our harvest outcomes. Although longer follow-up is desirable, our results regarding harvest success (92.6%) and ASCT outcomes, including engraftment and MM response at 100-days, are comparable to those reported by the CASSIOPEIA trial.
Disclosures
Sanderson:Novartis: Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal